If you need assistance meeting ClinicalTrials.gov obligations, the Indiana CTSI and the Office of Research Compliance (ORC) are here to help.
Services provided:
- The Indiana CTSI provides support and guidance to investigators and study record managers in meeting ClinicalTrials.gov obligations.
- The ORC administers the IU ClinicalTrials.gov Compliance Program.
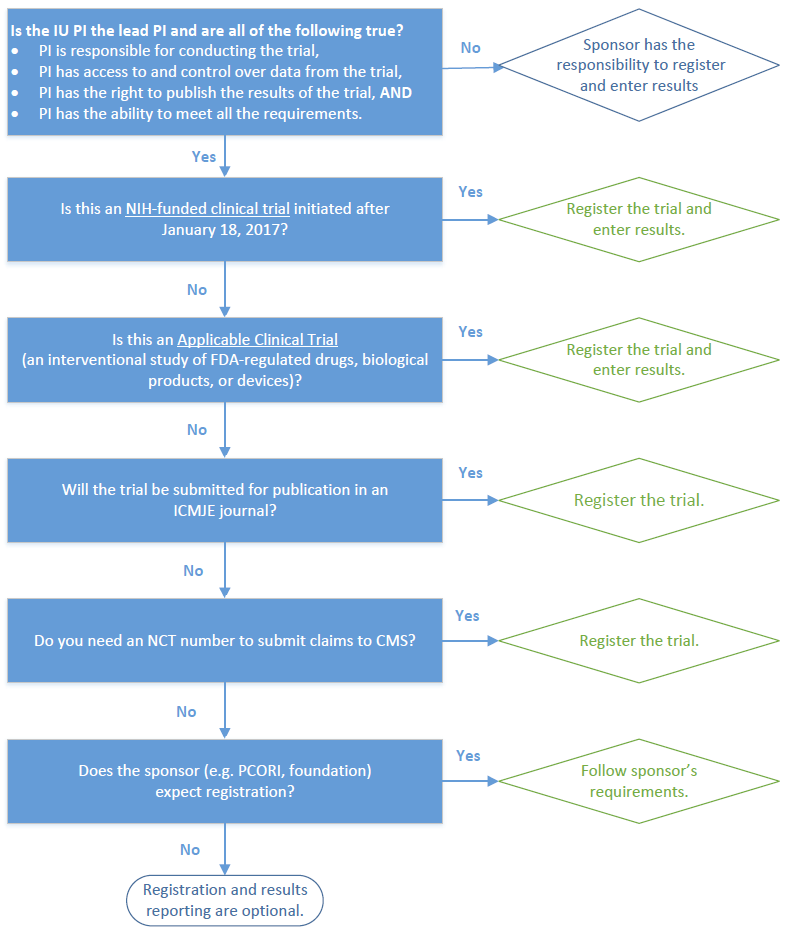
Do I need to register and post results?
NIH Grant Language
Grant template language where IU PI is Lead PI:
ClinicalTrials.gov Dissemination Plan
The principal investigator will abide by the NIH Policy on the Dissemination of NIH-Funded Clinical Trial Information. The investigator intends to register the trial, maintain and update the record, and submit results information to ClinicalTrials.gov, consistent with policy and regulatory requirements. The Indiana University Office of Research Compliance, in collaboration with the Indiana CTSI, offers a robust compliance program and provides guidance to investigators in meeting their policy and regulatory obligations.
Grant template language where IU is a site but IU PI is not the Lead PI:
ClinicalTrials.gov Dissemination Plan
The investigator intends to coordinate with the lead principal investigator to ensure that all regulatory and policy requirements are met in compliance with the NIH Policy on the Dissemination of NIH-Funded Clinical Trial Information. The investigator intends to provide necessary information to the lead PI to facilitate registering the trial, maintaining and updating the record, and submitting results information to ClinicalTrials.gov. The Indiana University Office of Research Compliance, in collaboration with the Indiana CTSI, offers a robust compliance program and provides guidance to investigators in meeting their policy and regulatory obligations.
Resources:
Training by ClinicalTrials.gov
Contact:
IU ClinicalTrials.gov Administrator at ctgov@iu.edu