The Indiana Clinical and Translational Sciences Institute (CTSI) provides a wide range of support services and funding for scientists at all stages of the translational research pipeline across its partner institutions of Indiana University, Purdue University and the University of Notre Dame.
For clinical and biomedical principal investigators (PI) in academia, the innovation and commercialization process can be a daunting departure from translational NIH and other extramurally funded research.
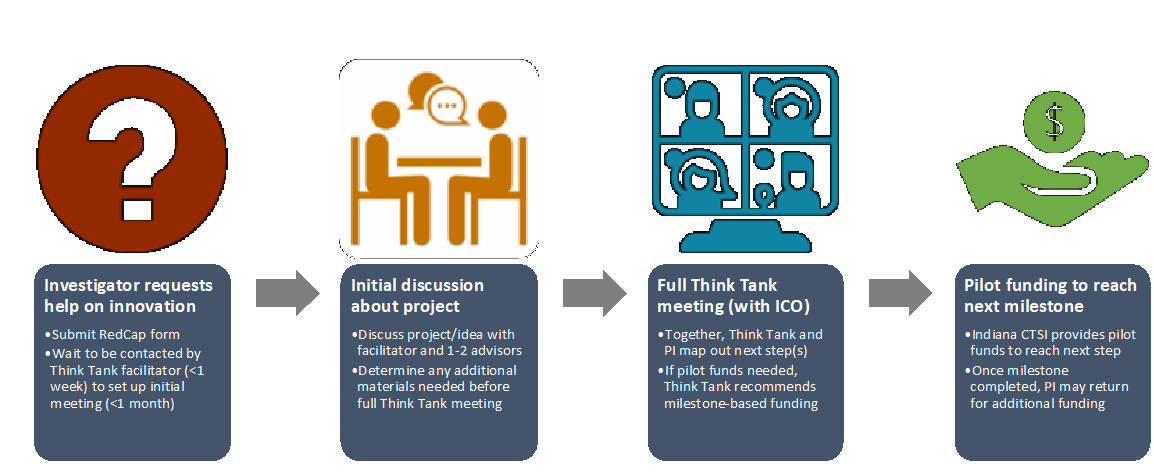
To support their efforts and to assist PIs along the path from discovery to commercialization, the Indiana CTSI is launching a Preclinical Innovation “Think Tank” pathway to help PIs advance their drug and device ideas to the market. The new program serves as a one-stop-shop where PIs can receive guidance from a pool of advisors – including experts in the drug and device industry – who serve as an essential resource for a wide range of scientific, technical, clinical, business, and regulatory questions. Projects will be guided from start to finish with robust tracking and milestone-based funding to help generate data for Investigational New Drug (IND) or Investigational Device Exemption (IDE) applications, small business grants such as the NIH Small Business Innovation Research (SBIR) and Small Business Technology Transfer (STTR) Programs, or commercialization.
For researchers working on new pharmaceuticals, Andrew Dahlem, PhD, chief of clinical pharmacology and senior research professor of medicine at IU School of Medicine and Richard Taylor, PhD, professor of chemistry and biochemistry at the University of Notre Dame and director of the Molecular Therapeutics Program for the Indiana CTSI, will co-lead the Think Tank pharmaceutical program.
For investigators working to commercialize innovative devices and software, Andrew Brightman, PhD, assistant head of biomedical engineering and associate professor of engineering practice at Purdue University and Jonathan Merrell, MD, assistant professor of clinical pediatrics at IU School of Medicine and deputy innovation officer for the Indiana CTSI, will co-lead the Think Tank device program.
“For a long time, the road to commercialization for many of our scientists has been a winding one. This think tank structure and its many talented leaders are going to straighten out that pathway and give our scientists the direction they need to start bringing their bright ideas to the masses,” said Sharon Moe, MD and co-director of Indiana CTSI.
Principal Investigators should submit this RedCap form to contact the Think Tank Navigators of Padma Portonovo, PhD, director of the Indiana CTSI Research Service Cores and Kara Garcia, PhD, assistant research professor of radiology and imaging sciences at IU School of Medicine Evansville campus, who will facilitate the pharmaceutical and device programs, respectively.
