Investigation of a Series of 1,4-diaryl-pyrazolo-pyridinones as Anti-Leishmanial Agents
Hannah Corman, Douglas A Shoue, Bruce J Melancon, Mary Ann McDowell
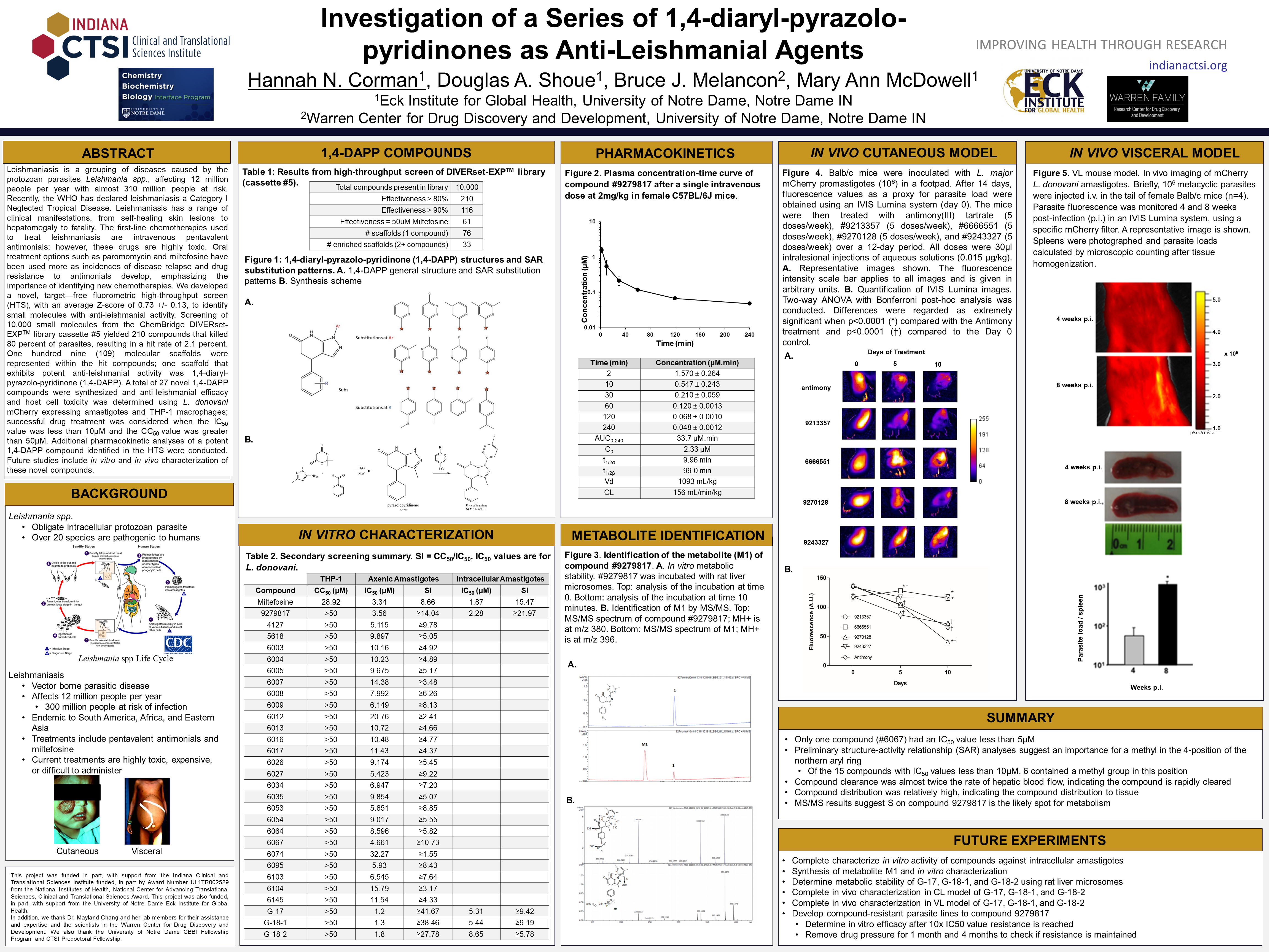
Impact: The first-line chemotherapies used to treat leishmaniasis are highly toxic intravenous antimonials yet drug resistance has begun to develop, causing the use of oral treatment options with high price tags; there is a strong need for new safe and effective chemotherapeutic agents to treat leishmaniasis.
Objectives/Goals: This study was conducted in order to identify novel chemical compounds that exhibit anti-leishmanial activity and to further characterize their efficacy and toxicity in in vitro and in vivo systems in the hopes of future chemotherapeutic developments.
Methods/Study Population: We developed a novel, target-free fluorometric high-throughput screen (HTS) to identify small molecules with anti-leishmanial activity. Screening of 10,000 small molecules from the ChemBridge DIVERset-EXP library cassette #5 yielded 210 compounds that killed 80% of parasites. One hundred nine (109) molecular scaffolds were represented within the hit compounds, including the 1,4-diarylo-pyrazolo-pyridinone (1,4-DAPP). A total of 27 novel 1,4-DAPP compounds were synthesized and anti-leishmanial efficacy and host cell toxicity was determined using L. donovani mCherry expressing amastigotes and THP-1 macrophages. Additional pharmacokinetic analyses of a potent 1,4-DAPP compound were conducted.
Results/Anticipated Results: Four experimental compounds had IC50 values less than 5μM, providing similar anti-leishmanial activity to miltefosine. Compound 9279817 had a clearance almost twice the rate of normal hepatic blood flow and had a relatively high volume of distribution, indicating this compound is rapidly cleared and distributes into tissues. In vitro rat liver microsome assays suggest a rapid metabolism of 9279817, and MS/MS results suggest this metabolite is most likely formed via oxidation of the sulfur on the lower aromatic ring.
Discussion/Significance of Impact: This study revealed a novel structural class of compounds that have anti-leishmanial activity. In vitro experiments show compounds with similar efficacy as miltefosine while having significantly less cytotoxicity, suggesting that this class could be further developed as a potential chemotherapeutic.