MedTech Commercialization PDT and Drug Development to Commercialization PDT
The skills and knowledge required for successful commercialization of new technologies (intellectual property protection, SBIR/STTR funding, and startup creation) are very different than those for traditional academic research (scientific publication and R01-style grant funding).
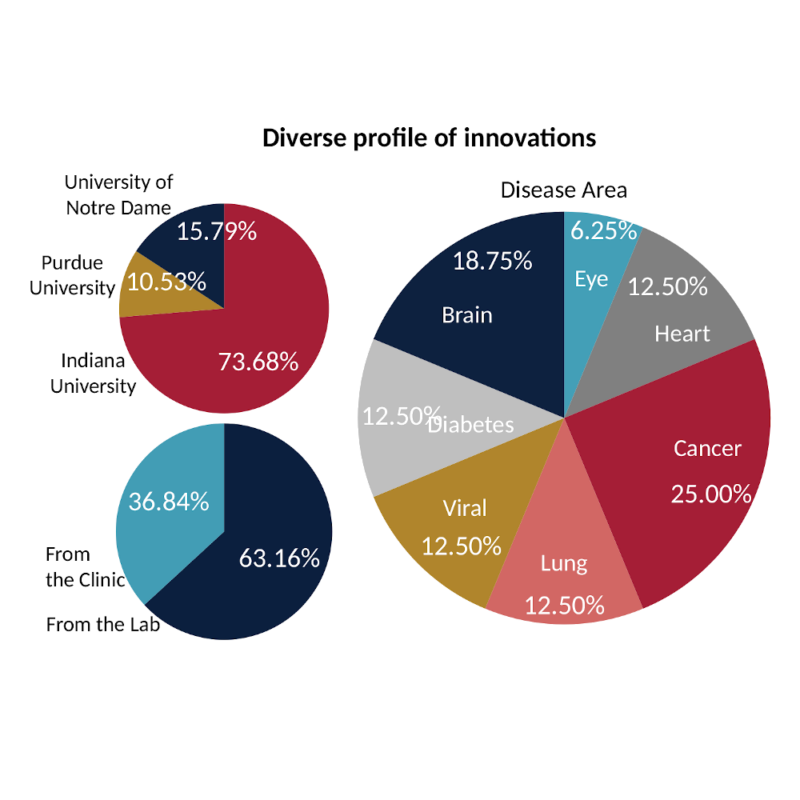
The Indiana CTSI MedTech Commercialization PDT and Drug Development to Commercialization PDT are designed to provide early guidance to academic and clinical investigators interested in advancing their discoveries to the market. The programs are open to investigators from Indiana University (IU), Purdue University, or the University of Notre Dame; and includes a pool of advisors across these universities and industry around the state to provide investigators with a wide range of expertise and perspectives.
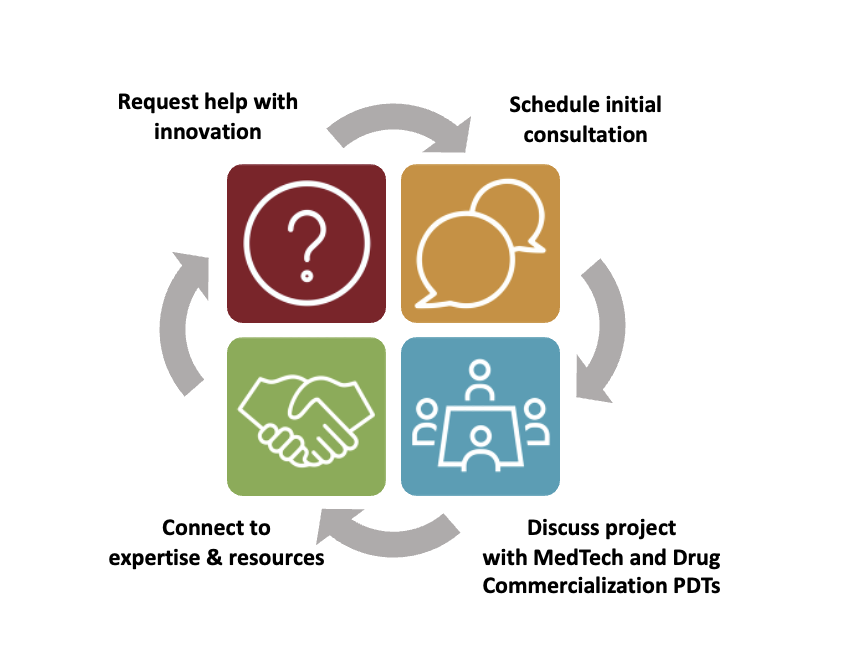
Services Provided
- Business and marketing advice
- Scientific and regulatory advice
- Connections to collaborators and industry partners
- Connections to relevant Indiana CTSI services and resources
- Connections to external services and resources
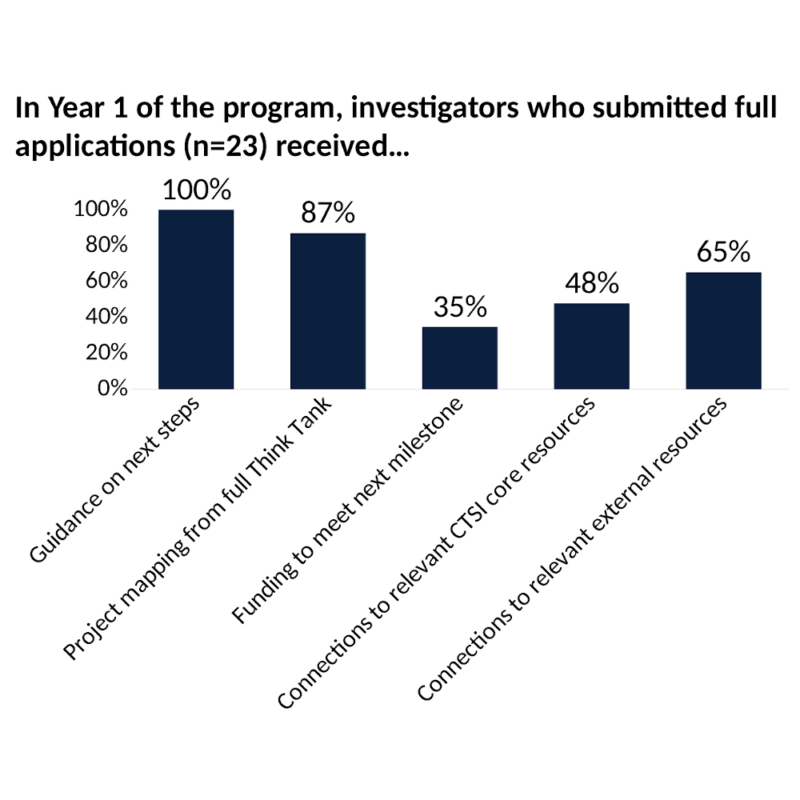
- Small-scale funding to meet next milestone
Drug development to commercialization projects: Padma Portonovo, PhD, Director of the IUSM Research Service Cores, Navigator for Drug PDT
pportono@iu.edu
MedTech commercialization related projects: Kara Garcia, PhD, Navigator for IU School of Medicine-Evansville (Southwest Indiana)
karagarc@iu.edu